Soil pH & soil type & fertilizer for no-spray own-root roses
strawchicago z5
2 years ago
last modified: 2 years ago
Featured Answer
Comments (130)
strawchicago z5
2 years agolast modified: 2 years agoslumgullion in southern OR
2 years agolast modified: 2 years agostrawchicago z5 thanked slumgullion in southern ORRelated Discussions
Own Roots, Soil, and Fertilizer in Florida
Comments (34)My most productive and rewarding activity over the past month has been murder. Yep, quite literally killing. And I must admit it gives me great pleasure to see the tiny brown specks which only the day before were an inconspicuous green. No longer sucking the life out of my rose buds, but in my imagination that life is drained out of them back into the roses. They are purely and plainly parasites. Aphids are to plants what fleas and ticks are to animals. And what pleasure it is! There's the challenge of the hunt. Without proper equipment (very good reading glasses) they'd escape detection. And then spritzing them with insecticidal soap! Ah, the joys of gardening. Similarly, the slight crunching sound as I squish the rose beetles under foot on the walk is somehow very very satisfying. As is the gentle search of myriad petals to find them nuzzled there. Gotchya! I understand the primates who preen their kin. And it's good practice for the next war. The one against the arrival of new fire ant queens who learn that they are sadly mistaken. My garden is NOT prime real estate for the likes of them....See MoreSoil pH = 7.7! Should I plant or cancel my order?
Comments (23)Thanks to everyone for all of the wise advice! I'm happy to see that last post by professorroush especially (thank you!) because I have decided to go ahead with the planting of the rugosas. At the very worst, I'll end up with a good post for the "worst gardening mistakes ever" forum ;) I do have a little bit of time to try and amend the area a little bit in the hope that I can add enough material to get the pH down a little bit at least. Do you all think I should mix in some peat? Or something else? Is there an acidic bagged soil widely available that is marketed for things like azaleas or anything like that? I love digging in the dirt so I'm not opposed to making it a bit of a raised/mounded bed either......See MoreYour plans for roses in ground and pots: soil prep & fertilizing?
Comments (105)anna, lavenderlace, aztcqn: There is something weird with this site due to which the posts do not show up in time. Most weird thing is that if I make a post from a new device (a new loptop, mobile phone etc), it is not visible even to me when I login from a different device. I can only see my own posts, immediately after posting, if I log on from the same machine..... I haven't understood how this happens. Feeding during monsoon has been a problem for me since past few years and I have tried different models. Since I wanted to be 100% organic, most solutions that I tried didn't work that well and my roses would stand exhausted and depleted by the end of monsoon. This year, I fed them after the monsoon in August and September with a doze that comprised 1 tea spoon each of Potash, gypsum, a water soluble 36-0-12 nitrogen fertilizer and a trace element supplement that contained Fe, Zn, Cu, Mn, Co and Mb mixed in 18 liters of water (capacity of the bucket). This had a good effect on the roses in pots that you can see in my threads of that time. There is also lot of discussion about the pros and cons of various methods... Fall Roses in Islamabad......zone 9b, October Roses.........zone 9b & September Roses, zone 9b Islamabad best regards...See MoreMeasuring pH in soil, compost and li: Need help calibating a pH meter?
Comments (13)Yeah ... lots of critical things to consider such as initial and changing pH effects*, buffering, multivalent cations, anionic and cationic micronutrients, and zeolite like ion exchange surfaces on soil particles, which make it a play day for chemistry discussions. Then the attack and breakdown of plant and animal litter to slow release nutrients brings up neat microbiology and biochemistry aspects. Material science then decides to manipulate the situation with osmotic release and diffusion of encapsulated nutrients. And hydroponic principles try to partially play nature taking over the hydrology and lighting it up. Physics kicks ideas in there on this last aspect. For instance did you know that fluorescent light indeed glow when struck by energy (as you know), but much of the light intensity flashes through a series of distinct colors at 60 times a second? * Plants cheat neatly by manipulating ion exchange release of cationic nutrients. They knock off ammonium, potassium, magnesium, calcium and other positively charged ions by producing acid(s) to knock it off. H+ alone can cause the exchange but if the acid is on a small organic base (anion) like oxalate this organic can diffuse around and pull at the cations on soil that the plant wants and help knock it off, helping in the "weathering" breakdown of soil too. Chelators made by plants and microbes make it really interesting too. They diffuse around and hold certain critical nutrients so tightly that the plant has the choice of finding more, having a deficiency (specific nutrient starving), making a stronger chelator to take it back, and/or breaking the chelator down to free the nutrient. Now the fun parts ... the plant one might be considering is not be alone. A group of similar or different roots might be working together AND competing in that patch of soil, with different players at different depths Trees cheat and certain non woody plants cheat and go low. Moles, gophers and field mice run through this soil zone toox playing their games. No soil contact then no nutrient uptake, no root then no nutrient collection there for the plant, loss of stored nutrients and need to spend energy replacing the root. And there are smaller life forms co-inhabating the soil with the roots that are also directly or indirectly effected by soil pH. Let's put them into three classes as those that (1) don't generally effect a plant much, (2) can hurt the plant, or (3) can help the plant. Let's see ... hurting a plant is bad, unless it hurts a seriously competitive plant more. Helping a plant is good, unless it's again that serious competitor. Plants are not stand alone organisms in naturem. They live in community with microorganisms. So what if the soil pH helps support the growth of a microbe that can grow all over your plants roots? Sounds bad I know, but there are those three classes mentioned above. If your microbe is a pathogen that is bad. If it doesn't attack the plant but runs out and breaks down nearby leaf litter, great free food. It it doesn't hurt your plant but by being on root surfaces can compete with and stop pathogenic microbes from getting a foothold, great a free natural inoculation for immunity. There was a company called Eden Bioscience a couple decades ago here in the PNW that made an interesting observation. In large scale evergreen seedling production for forestry sometimes there were large scale fungal blights. Sadly alot of the seedlings all died at once in mass. However, sometimes there were a few seedlings near each other that did not succumb! In fact they looked totally healthy! When isolating bacteria, yeast and filamentous fungi from the surfaces of these plants they found that certain kinds could be grown in the lab that protected seedlings from attack, when sprayed onto them. These microbes grew best in their optimal pH range. They indeed colonized the plants, in this case leaf surfaces. And their presence did protect the leaves from pathogen attack. Obviously similar things must be happening in nature in the leaf canopy and also soil root zone of plants. So when a plant likes acidic pH 5 - 6 soil, is this just because nutrients are more available it? When I went to school, in what now seems like the dark ages, most plant physiology books focused almost solely on this. Or is it because beneficial microbes helping feed or protect the plant need that pH? My firm assumption is that both chemical and microbial pH dependent effects interact to make an optimum environment for that plant. And that some plants in the natural environment survive best, rather than grow best, at their optimal pH range. Why do many fungi sour (strongly acidify) what they are busy rotting? Niether competive microbes nor does the dieing plant tissue like it. The fungus gets more. This is exactly why you want to check the pH in the soil that you might be soon preparing for your new vegetable or herb garden this spring. Too basic, your plants starve. Too acidic, the pathogenic fungi don't starve. Then like the heirloom story of The Three Bears ... there's one pH that's just right....See Morestrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agorosecanadian
2 years agostrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agoVaporvac Z6-OhioRiverValley
2 years agolast modified: 2 years agostrawchicago z5 thanked Vaporvac Z6-OhioRiverValleystrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agoann beck 8a ruralish WA
2 years agolast modified: 2 years agostrawchicago z5 thanked ann beck 8a ruralish WAstrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years agolast modified: 2 years agostrawchicago z5
2 years ago
Related Stories

FARM YOUR YARDHow to Get Good Soil for Your Edible Garden
The nutrients in your soil feed the plants that feed you. Here are tips on getting it right — just in time for planting season
Full Story
CONTAINER GARDENSContainer Gardening Basics: The Dirt on Soil
Learn the types of potting soil available and the best mixes to help your containers thrive
Full Story
GARDENING GUIDESHow to Stop Worrying and Start Loving Clay Soil
Clay has many more benefits than you might imagine
Full Story
WINTER GARDENINGHow to Plant Bare-Root Roses
Late winter or early spring is a great time to put new roses into the ground
Full Story
GARDENING GUIDESGardening Solutions for Heavy Clay Soils
What’s a gardener to do with soil that’s easily compacted and has poor drainage? Find out here
Full Story
GARDENING GUIDESHow to Pick a Mulch — and Why Your Soil Wants It
There's more to topdressing than shredded wood. Learn about mulch types, costs and design considerations here
Full Story
GARDENING GUIDESGet on a Composting Kick (Hello, Free Fertilizer!)
Quit shelling out for pricey substitutes that aren’t even as good. Here’s how to give your soil the best while lightening your trash load
Full Story
SPRING GARDENINGHow to Grow a Rose Garden in Pots
Everything can come up roses, even without a plot of soil in sight. This step-by-step guide to growing roses in containers shows you how
Full Story
GARDENING GUIDESLearn the Secret to Bigger and Better Roses
Grow beautiful roses using both ordinary and unusual soil amendments
Full Story
EDIBLE GARDENSHow to Grow Your Own Luscious Cherries
Nope, they’re not the easiest fruit to grow. But with spectacular blossoms and pies as possibilities, cherries are sure worth a try
Full Story
















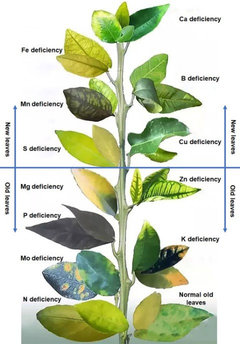

























strawchicago z5Original Author